
Non-metals are stronger than metals and can get electrons very easily from metals. They are formed between a metal(+ion) and a non-metal (-ve ion).
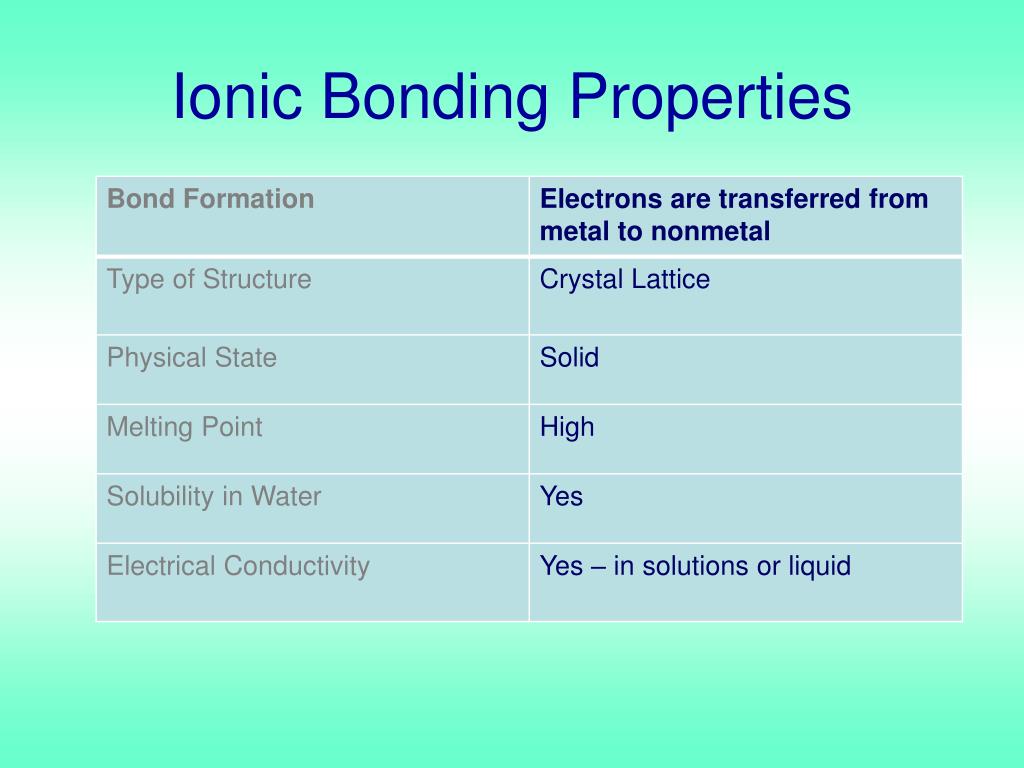
For stabilization, neighbouring they share their electrons from the outermost orbital. Neither of the atoms is strong enough to attract electrons from the other. They are formed between two non-metal having similar electronegativities. Non-polar covalent compounds are soluble in organic liquids only.īeing polar, ionic compounds are soluble in polar solvents only like water. Polar covalent compounds dissolve in polar solvents. Ionic compounds are made up of oppositely charged particles called cations and anions. It consists of electrically neutral discrete molecules Two non-metals or a non-metal and a metalloid It is a form of chemical bonding between two non-metallic atoms, which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds.Īlso known as an electrovalent bond, it is a type of bond formed from the strong electrostatic force of attraction between oppositely charged ions in a chemical compound. (a) The number of valence electrons present in the atoms involved in bonding -.Ītoms possessing one, two, or three valence electrons which are positioned in groups \( \right).\) Various factors affect the formation of these ions and consequently ionic bonding, which ultimately gives rise to ionic compounds. We also know that chemically, table salt is sodium chloride in which sodium and chloride ions are bonded together by ionic bonds.

We are all quite familiar with table salt that we use to add flavour to food. In ionic compounds, atoms need to fulfil several conditions to form an ionic bond.


 0 kommentar(er)
0 kommentar(er)
